
How to Use Hydrochloric Acid for Professional Cleaning
Table of Contents
- What Is Hydrochloric Acid and When to Use It in Cleaning
- Essential Personal Protective Equipment (PPE) for Handling HCl
- Step-by-Step Guide: Safe Dilution and Application
- Professional Storage, Transport, and Disposal Protocols
- Hydrochloric Acid Alternatives: When to Choose a Different Cleaner
- Your Professional Standard for Hydrochloric Acid Safety
- FAQs
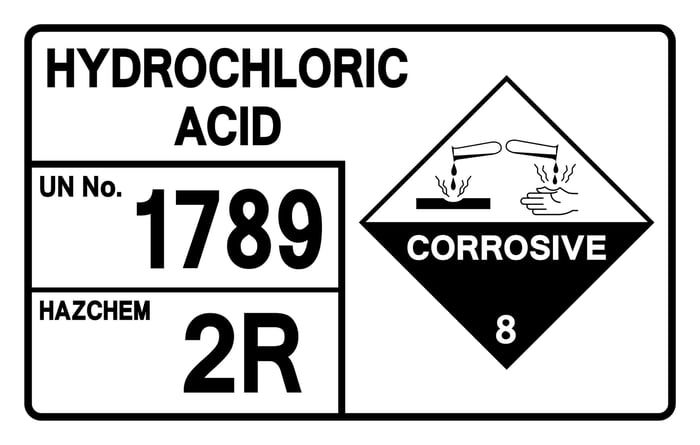
For the professional exterior cleaner, few chemicals command as much respect—and create as much uncertainty—as hydrochloric acid. Understanding how to use hydrochloric acid correctly is critical, as improper application can cause irreversible damage to a client’s property and lead to serious personal injury. When applied with precision and expert knowledge, however, it becomes an unparalleled solution for removing the toughest stains on masonry and concrete. That gap between a costly mistake and a perfect result is where professional procedure makes all the difference.
The constant concern over proper dilution ratios, essential PPE, and safe application techniques can be a major barrier to taking on heavy-duty cleaning jobs with confidence. This definitive guide eliminates that uncertainty. We provide the essential, step-by-step instructions for safely handling, diluting, applying, and storing muriatic acid. You will learn the correct protocols to protect yourself and your team, master the techniques for removing stubborn efflorescence and rust, and gain the expert knowledge to know exactly when this powerful acid is the right tool for the job.
What Is Hydrochloric Acid and When to Use It in Cleaning
WARNING: Hydrochloric acid is an extremely hazardous and corrosive chemical that can cause severe injury, chemical burns, and respiratory damage. It must be handled only by trained professionals equipped with appropriate Personal Protective Equipment (PPE), including acid-resistant gloves, full-face shields, and respiratory protection. This guide is for informational purposes for industry professionals and does not replace formal safety training.
Hydrochloric acid (HCl) is a powerful, high-performance mineral acid used for the most demanding industrial and professional cleaning jobs. While often used interchangeably with muriatic acid, it's important to note that muriatic acid is a less pure, commercial-grade form. For a detailed technical overview, you can explore this resource on what is hydrochloric acid and its chemical properties. Due to its aggressive nature, this chemical should be considered a last-resort solution, reserved for stubborn contaminants when all other professional-grade cleaners have failed.
To see its powerful effects on rust, watch this brief demonstration:
Common Applications in Professional Exterior Cleaning
When used correctly by a trained professional, HCl delivers exceptional results for specific, challenging jobs on durable masonry surfaces. Its primary function is to dissolve mineral-based deposits and prepare surfaces for further treatment.
- Efflorescence Removal: Effectively dissolves the white, powdery salt deposits that appear on brick, block, and concrete surfaces.
- Concrete Etching: Prepares new, smooth concrete for sealers, paints, or epoxy coatings by creating a porous profile for superior adhesion.
- Masonry Brightening: Removes deeply embedded dirt, mortar smears, and atmospheric stains from hard-fired brick and other non-sensitive masonry.
- Rust and Mineral Stain Removal: A heavy-duty solution for breaking down tough metallic and mineral stains on concrete and other durable surfaces.
Surfaces to AVOID Using Hydrochloric Acid On
This chemical's corrosive power will cause irreversible damage to acid-sensitive materials. The acid reacts with calcium carbonate in stones like limestone and marble, dissolving the surface and causing permanent etching and degradation. Always test on a small, inconspicuous area before full application. Do not use on:
- Limestone, marble, and travertine
- Polished stone or granite
- Most metals (can cause severe corrosion and discoloration)
- Asphalt, vinyl siding, and certain plastics
Essential Personal Protective Equipment (PPE) for Handling HCl
When handling a powerful chemical like hydrochloric acid, your standard work gear is not just inadequate—it's a liability. Professional-grade Personal Protective Equipment (PPE) is not optional; it is a mandatory requirement for safe operation. This gear is specifically designed with chemical-resistant materials to protect you from corrosive splashes and toxic fumes. Before every job, a thorough inspection of all PPE for cracks, holes, or degradation is an essential, non-negotiable step.
Respiratory and Eye Protection
Protecting your eyes and lungs is the first priority. Inhaling HCl fumes can cause immediate and severe damage to your respiratory system. The risk of pulmonary edema—a life-threatening condition where fluid fills the lungs—is significant, as detailed in resources like the NJ Health Hazardous Substance Fact Sheet. A full-face respirator equipped with acid gas (AG) cartridges is mandatory. For eye protection, this respirator provides a complete seal, but ANSI Z87.1-rated chemical splash goggles are the absolute minimum requirement.
Skin and Body Protection
Direct contact with hydrochloric acid causes severe chemical burns. Your entire body must be shielded with appropriate, non-reactive materials. Standard cotton or leather gear will degrade on contact and offers no real protection. Your essential body protection includes:
- Acid-Resistant Gloves:Heavy-duty neoprene or nitrile gloves are required. For jobs requiring more dexterity, a layered approach with nitrile over latex can be effective.
Natural Rubber Gloves

$9.00
Natural Rubber Blue Grip Gloves Blue Latex-Free PowerhousePremium natural rubber with cotton knit lining, etched non-slip grip, and 100% waterproof barrier — chemical, acid, hot...… read more
- Chemical-Resistant Boots:Full-length rubber or PVC boots are essential. Leather work boots are completely unsuitable as acid will quickly destroy them.
PVC White Boot

$41.30
Boss White Majesty 16" PVC Knee Boots Assembled in USA • Waterproof • Chemical-Resistant16" over-the-sock PVC knee-high boots with Safety Loc non-slip sole, steel shank,...… read more
- Full Body Suit: A PVC or rubber rain suit provides excellent splash protection. For more intensive applications, a full, professional-grade acid-resistant suit is the recommended solution.
Investing in the right gear is a critical part of any professional operation. Shop for professional-grade safety equipment here.
Emergency Preparedness
Your on-site safety plan must include immediate access to emergency equipment. This is a crucial extension of your PPE, designed to mitigate exposure the moment it happens. Ensure your work area is equipped with:
- A portable eyewash station kept within immediate reach of the handling area.
- A readily available neutralizing agent, such as a container of baking soda or garden lime, to manage small spills.
- A reliable source of running water for emergency flushing of skin or equipment for a minimum of 15 minutes.
Step-by-Step Guide: Safe Dilution and Application
Executing a job and understanding how to use hydrochloric acid demands a methodical, controlled process. This is not a task for speed; it is a task for precision. A professional approach minimizes risk and maximizes results. Before you begin, always assess the worksite for environmental factors. Pay close attention to wind direction to prevent drift onto non-target surfaces, and take proactive steps to protect surrounding vegetation, vehicles, and adjacent property from any contact with the chemical solution.
Preparation and Mixing
Proper preparation is the foundation of a safe and effective application. Before mixing, pre-wet the target surface and any nearby plants with a copious amount of water. This initial saturation prevents the acid from absorbing too quickly and protects foliage from chemical burns. Your mixing equipment must be professional-grade.
- Use only a heavy-duty, acid-resistant plastic bucket. Never use metal containers.
- Begin with a standard dilution ratio of 10 parts water to 1 part acid (10:1). Adjust only if necessary and with extreme caution.
- Follow the essential ‘Three A’s’ rule: Always Add Acid to water. Slowly and carefully pour the acid into the water to prevent a violent, exothermic reaction that can cause splashing and eruption.
Application and Dwell Time
Control is paramount during application. Work in small, manageable sections (e.g., 10x10 feet) that you can thoroughly rinse before the solution dries. This prevents the acid from leaving streaks or causing damage to the surface.
- Apply the diluted solution evenly using a plastic, acid-resistant pump-up sprayer.
- WARNING: Never introduce an acid solution into a pressure washer pump or its components. Doing so will cause catastrophic failure of seals, O-rings, and internal parts.
- Let the solution dwell on the surface for only 2 to 5 minutes. Do not allow it to dry under any circumstances.
Rinsing and Neutralization
Once the dwell time is complete, immediate and thorough rinsing is critical to stop the chemical reaction. The goal is to completely remove all acidic residue from the surface and surrounding areas.
- Rinse the area with a high volume of fresh water. Start at the top and work your way down.
- For sensitive surfaces or to ensure complete safety, follow the initial rinse with a neutralizing solution, such as a mixture of baking soda and water.
- Ensure all runoff is controlled and managed according to local environmental regulations.
Professional Storage, Transport, and Disposal Protocols
As a professional, your responsibility for a chemical like hydrochloric acid extends far beyond its application. Proper handling throughout its entire lifecycle—from the moment it arrives at your shop to its final disposal—is a critical part of your job. Failing to follow established safety and legal protocols not only endangers you and the public but also exposes your business to heavy fines and liability for environmental damage. These guidelines are not suggestions; they are essential operational standards.
Safe Storage and Transportation
Secure handling at your facility and on the road is fundamental to professional chemical management. In your shop or truck, always adhere to these core principles to prevent accidents, ensure regulatory compliance, and maintain a safe work environment.
- Original Containers: Always store acid in its original, clearly labeled, and tightly sealed container. Never transfer it to an unmarked bottle.
- Proper Location: Keep containers in a cool, dry, and well-ventilated area away from direct sunlight and incompatible materials like bases (e.g., sodium hypochlorite), metals, and oxidizers.
- Secure Transport: When transporting chemicals, secure them upright within a secondary containment bin to prevent tipping and contain any potential leaks.
- Safe Placement: Never store acid on a shelf above eye level. This minimizes the risk of it being dropped or splashing onto your face and body.
Spill Containment and Cleanup
Accidents can happen, but a professional is always prepared. A rapid and correct response is crucial to mitigating harm. Every work vehicle carrying acid should be equipped with a dedicated acid spill kit containing personal protective equipment (PPE), a neutralizer, and absorbent materials. If a spill occurs, first neutralize the acid with an appropriate base like soda ash or baking soda. Once the fizzing stops, use the absorbent pads to contain and collect the residue. All cleanup materials must be bagged and disposed of as hazardous waste.
Proper Disposal of Unused Acid
Improper disposal of unused hydrochloric acid is illegal and environmentally irresponsible. Never pour it down a storm drain, into a sink, or onto the ground, as this can contaminate water supplies and harm ecosystems. Your legal obligation is to contact your local or regional hazardous waste disposal facility. They will provide specific instructions for drop-off or collection. While some local regulations may permit neutralizing very small, diluted amounts for disposal, you must verify and strictly follow these specific municipal codes first.
Hydrochloric Acid Alternatives: When to Choose a Different Cleaner
At J Racenstein, our goal is to equip professionals with the right tool for every job. While a powerful mineral stain remover, hydrochloric acid is not a one-size-fits-all solution. A core principle of professional exterior cleaning is to use the least aggressive chemical necessary to achieve the desired result. This approach protects the substrate, ensures safety, and delivers superior outcomes. Knowing when to choose an alternative is critical for any serious contractor.
For Organic Stains (Algae, Mildew)
Acids are fundamentally ineffective against organic growth like algae, lichen, and mildew. These stains require an oxidizing agent to be properly neutralized and removed. For these applications, a professional-grade sodium hypochlorite (bleach) based cleaner is the correct choice. When combined with high-performance surfactants, these cleaners can cling to vertical surfaces, penetrate deeply, and deliver a complete kill of the growth for long-lasting results. ProTool stick was developed to be used with sodium hypochlorite to allow proper dwell time to kill organic growth and mildew.
ProTool Sticky

$66.10
ProTool Sticky Super Concentrate The industry’s highest-concentration sticky surfactant — gives maximum cling, superior spreading, and extended dwell time when used with sodium hypochlorite (SH)....… read more
For General Grime and Degreasing
When tackling heavy buildup of oils, grease, and carbon-based soils, an acid is the wrong tool. These situations call for powerful alkaline cleaners or professional degreasers. These products work by lifting and emulsifying oily grime, allowing it to be easily rinsed away. They are the go-to solution for demanding commercial jobs like cleaning gas station concrete pads, restaurant drive-thrus, and equipment maintenance bays.
EBC Degreaser, Detergent

$222.55
EBC Degreaser, Detergent When Nothing Else Cuts It – Enviro Bio Cleaner (EBC) works hard so you don't have to. This heavy-duty cleaner is the...… read more
For Other Mineral Stains (Rust)
Even when an acid is required for mineral stains, hydrochloric acid should not always be the first choice. For targeted issues like rust stains on concrete or brick, less aggressive options are often safer and just as effective.
- Oxalic Acid: The industry standard for effectively removing rust stains without the high-fuming risk of stronger acids.
- Phosphoric Acid:A versatile option for removing efflorescence, hard water stains, and metal oxides with a more controlled reaction.
Front9 Restoration Rust Remover F9 B.A.R.C.

$2,140.05
F9 BARC: The World's Best Rust Remover Banish rust stains for good with F9 BARC—the world’s best rust remover, engineered to erase orange battery acid...… read more
Starting with these specialized acids minimizes the risk of surface damage and is a safer first step in your stain removal process.
Matching the chemical to the contaminant is the mark of a true professional. Building a versatile chemical arsenal ensures you are prepared to tackle any job safely and efficiently. Explore our full range of professional cleaning chemicals.
Your Professional Standard for Hydrochloric Acid Safety
Handling powerful chemical solutions is a core competency for any serious cleaning professional. This guide has established three critical principles: first, reserve hydrochloric acid for appropriate, heavy-duty applications where its strength is truly required. Second, the use of professional-grade Personal Protective Equipment (PPE)—including acid-resistant gloves, splash-proof goggles, and respirators—is absolutely mandatory, not optional. Finally, strict adherence to established protocols for dilution, application, and disposal is essential for ensuring safety and achieving superior results on the job.
At J. Racenstein, we support professionals who demand performance and reliability. As the industry's one-stop shop, we offer more than just an extensive inventory of cleaning equipment; we provide the expert advice and durable, high-performance safety gear you need to work with confidence. From fall protection to chemical-resistant apparel, we have the solutions to keep you protected. Browse our complete collection of professional safety gear and cleaning equipment.
Equip yourself for excellence and tackle every challenge with the right tools and knowledge.
FAQs
What is the difference between hydrochloric acid and muriatic acid?
Muriatic acid is a less-pure, diluted grade of hydrochloric acid. While chemically the same (HCl), muriatic acid typically has a concentration of around 31.5% and contains impurities like iron, which give it a yellowish tint. It is commonly used for professional tasks like cleaning concrete and brick. In contrast, reagent-grade hydrochloric acid is purer and used in laboratory settings. For most exterior cleaning applications, muriatic acid is the standard product.
What should I do immediately if I get hydrochloric acid on my skin?
Immediate action is critical. If you get hydrochloric acid on your skin, flush the affected area with large amounts of cool, running water for at least 15-20 minutes. While flushing, carefully remove any contaminated clothing or jewelry. Do not attempt to scrub the area or apply any ointments. After thorough rinsing, seek immediate professional medical attention to assess the severity of the chemical burn and receive proper treatment.
Can you put hydrochloric acid in a pressure washer?
No, you should never put standard hydrochloric (muriatic) acid in a pressure washer unless the system is specifically designed for acid application. The corrosive nature of the acid will rapidly damage critical internal components, including the pump, seals, O-rings, and wand. This equipment failure can cause dangerous leaks and high-pressure spray of acid, creating a significant safety hazard. Only use professional-grade, acid-resistant injectors and application systems for such tasks.
Is it safe to mix hydrochloric acid with bleach?
Absolutely not. It is extremely dangerous to mix hydrochloric acid with bleach (sodium hypochlorite). This combination creates a chemical reaction that releases toxic chlorine gas. Inhaling chlorine gas can cause severe respiratory damage, chemical burns to the lungs, and can be fatal, even with minimal exposure. Always keep these chemicals separate and ensure adequate ventilation when working with either product individually to prevent accidental mixing of fumes.




